The Keiderling Group
Research
The Factor Analysis methods we used for protein spectral interpretation in terms of fractional secondary structure were developed by Petr Pancoska in the 1990s using the Famulus language, which is not readily available, hence the programs are no longer supported by our group (i.e. me!). They work essentially the same as a commercial software package available from BioTools. However the original data sets in principle should be useful with any spectral analysis package, and should be available here in a generic format for IR, VCD and ECD with H2O and D2O for up to 28 proteins of different structural types. The data are now 30 years old, and the links are not always reliable, but whatever you can download is free!
Program, ECD, IR-H2O, IR-D2O, VCD-H20, VCD-D2O
FARMR
Current research areas
Biomolecular Structure and Folding Studies
Our research has primarily been focused on the use of spectroscopic techniques to determine biomolecular structure and follow its dynamic changes. Our group has been recognized for developing new IR and vibrational circular dichroism (VCD) based optical spectroscopic methods and applying them to the study of peptide and protein conformation and folding. Through collaborations, we have developed new applications of ab initio QM methods to model and interpret our experimentally obtained peptide IR, Raman and VCD spectra. Our UIC spectral studies probed equilibrium and thermodynamic changes for peptides and proteins, as well as used collaborative studies of microsecond dynamics with IR-detected T-jump methods.



Peptide Studies
In the past two decades, our emphasis has been on the use of model peptide systems to exemplify fundamental secondary structure types and to model properties of small folding units in proteins. With site-specific isotopic labeling we have been able to use these low resolution techniques to obtain residue specific structure and dynamics.
Our studies have been enhanced by collaborations using NMR structure determination to generate models for folded structures and MD methods to develop models for folding processes and intermediate structures on the pathway.

More recently we undertook studies related to protein and peptide aggregation and fibril formation, processes important in many biomedical conditions. Unique IR and VCD spectral patterns can be correlated with TEM images to follow fibril formation under various conditions.
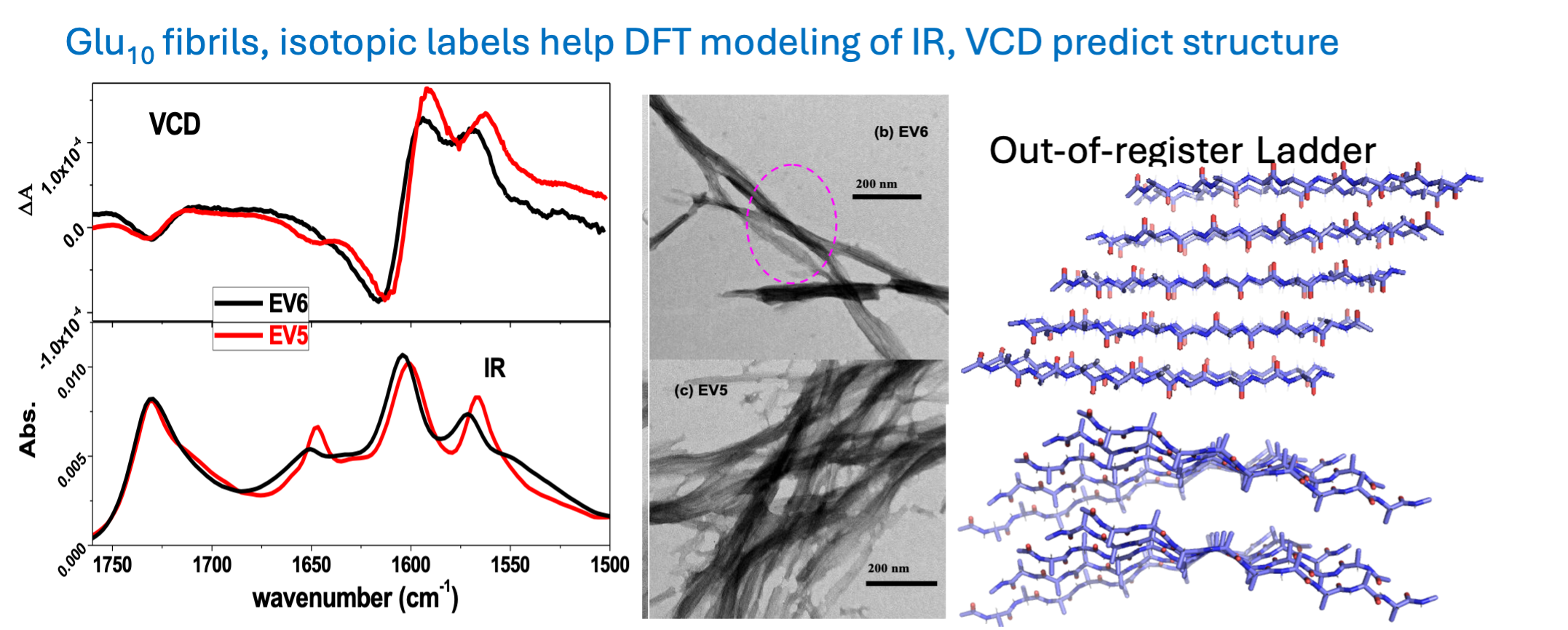
Protein Studies
Previously, the group studied folding of larger proteins, with an emphasis on b-sheet dominant structures, aggregates, and models for protein membrane interaction and folding studies. Proteins were unfolded or induced to form new structures by perturbation effects on protein and protein complex structures, such as disulfide reduction, surface binding, pH and thermal variation. Additionally protein-lipid vesicle binding examples were studied with equilibrium and dynamic (stop-flow) IR, CD and fluorescence methods



Previous Protein Secondary Structure
Many spectroscopic properties reflect with protein secondary structure, either by band shape or transition frequencies, and VCD is no exception. A number of different algorithms were developed primarily by Petr Pancoska to determine average content based often on statistical methods corelating band shape patterns. We originally extended such methods to include VCD, and additionally did coupled analyses of VCD and IR and ECD to get better statistical fits to structure. Our local method was termed FARMR, a restricted factor analysis regression, and includes several data bases which are available, hopefully linked below and explained in many of our papers in the 1990s.



Nucleic Acids
These same IR, VCD, and ECD methods were also used to study DNA and RNA equilibrium folding and stability with variations in temperature and ionic strength as the primary perturbations.

Other Studies
Small Molecule Studies

Our initial research efforts at UIC emphasized VCD studies of small molecules, emphasizing organic ring models made chiral by isotopic substitution for purposes of theoretical modelling.
Transition Metal complex spectral studies
Magnetic CD (MCD) studies of heavy metal complexes utilizing our near-IR MCD capabilities were initially undertaken for tests of ligand field theory. Additional studies were made using two-photon excited fluorescence and ionization of d-d transitions.

Molecular Zeeman
Finally a number of studies were done to explore the first measurements and develop theoretical models of Magnetic VCD, or the Zeeman effect for molecular vibrations, for both solution and gas phase samples of selected high symmetry small molecules.


We were part of a large project/grant to develop methods of detection biomarkers for laser-induced eye injuries. Our method used surface enhanced Raman spectra to detect labeled nanoparticles that were complexed to the fixed biomarkers with antibody technologies (ELISA type assay). When funding ended, we left the project.